By Jonathan Cors
Several years ago a previous member of our lab, Garett Heineck, wrote an article explaining Round-up Ready Kentucky bluegrass, which was the first release of a turfgrass that was genetically modified to have glyphosate herbicide resistance. The article does a great job explaining genetically modified turfgrass, as well as its uses, issues, and why it’s largely not currently sold.
One major topic of his article was the idea of unintentional spread of genetically modified genes. This is an issue with all outcrossing genetically modified crops, as they can spread their genetic mutations to any offspring during cross pollination with other plants, including non-genetically modified plants where the genes may not be wanted. However, even though this is a large issue, genetically modified agronomic crops are still grown all over the world. Farmers deal with this gene spread issue in a very simple way. As long as their crops are grown an approved distance away from any other farmer growing the same crop, there is less concern of contamination. The pollen can only travel a certain distance, so the farmers make sure they are outside that distance.
Due to the prevalence of turfgrasses on many types of landscapes, if genetically modified turfgrass was made, it would likely spread that genetic modification; this would especially be a concern in regions where turfgrass seed is produced. This issue is why there is limited genetically modified turfgrass on sale in the market and also why the growing toolsets in plant molecular biology go largely unused in turfgrass. The project I will be running as a new graduate student is centered around addressing this issue of unintended gene spread by utilizing a genetic construct developed in my co-advisor’s lab, Dr. Michael Smanski, called engineered genetic incompatibility (EGI), which has previously been proven to work in the model organism Drosophila melanogaster, commonly referred to as the fruit fly.
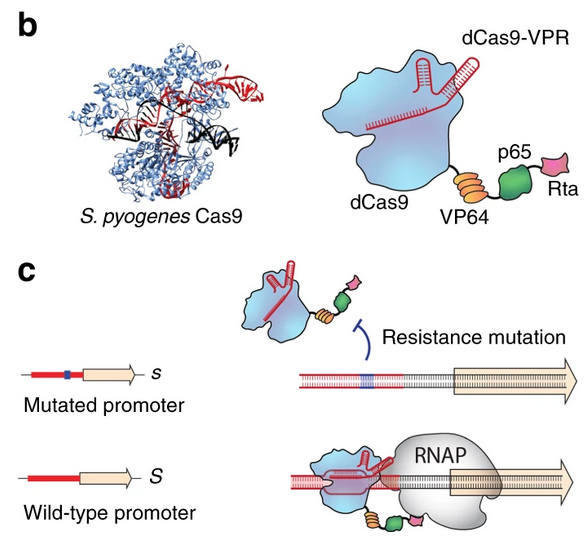
To explain how EGI works, I will be utilizing a paper and some of its figures that was previously published by members of Dr. Smanski’s lab. Figure 1 showcases the main components of EGI and how it works. A CRISPR-activator, referred to as dcas9-VPR in the paper, is an enzyme that can be designed to go to the beginning part of a gene, the promoter region, and subsequently cause overexpression of that gene. If you do this with a gene that is tightly regulated in an organism, that overexpression will cause the organism to die. An EGI organism has two mutations. First, a gene encoding a CRISPR-activator designed to recognize the DNA sequence for the wild-type promoter, meaning the sequence that non-genetically modified organisms will have. Second, a small mutation that changes the sequence of the promoter region for our gene target within the EGI organism. These two mutations together mean that while the CRISPR-activator is made in an EGI organism, since its target promoter region has been mutated, it has nothing to bind to and nothing about the EGI organism is phenotypically different.
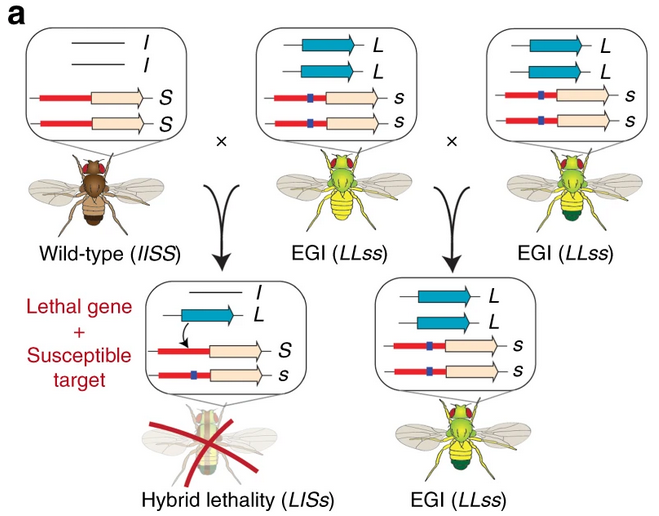
However, while an EGI organism may not look or act differently from non-genetically modified organisms, Figure 2 showcases what happens when an EGI organism mates with a non-EGI organism. The hybrid offspring will now have one copy of the gene that encodes the CRISPR-activator, and one copy of the non-mutated promoter. This gives the CRISPR-activator a target, leading to overexpression, and to hybrid lethality.
EGI has many uses dependent upon the organism it’s inserted into, but all of them center around population control. For instance, continuing the example of insects, an EGI mosquito could be released into the environment to mate with the wild-type population, causing all the hybrid offspring to die. This would decrease the overall population size with several releases of EGI mosquitos. Utilizing the idea in plants, EGI is a way to create a genetically modified turfgrass unable to breed with any non-genetically modified turfgrass, solving the uncontrollable gene spread problem.
With this problem solved by EGI, the worry of uncontrolled genetic modifications in turfgrass would no longer exists. Further genetic modifications that provide an actual benefit could be introduced into an EGI turfgrass. Many of the genetically modified traits that agronomic crops utilize such as stress tolerance and disease resistance can then be used in turfgrass, creating cultivars with traits that are better able to withstand a changing climate.